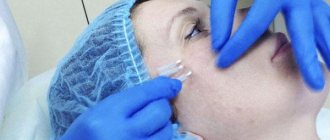
About the procedure
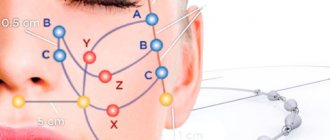
The peculiarity of the technique is the technique of introducing fillers. The doctor injects the drug not volumetrically, but “channelwise,” along certain lines, thus forming a network of thin microtunnels filled with the drug.
Over the course of 8-12 months, the mesh will completely dissolve, and in its place will be formed its own collagen frame made of natural fibers, which not only keep the tissue from sagging and create a visible tightening effect, but also prevent the appearance of new folds and wrinkles.
Vector facial skin lifting is performed with Restylane and Radiesse.
Restylane is a filler based on high-density hyaluronic acid. When administered, it accumulates water molecules, creating volume and eliminating wrinkles. The drug contains only hyaluronic acid, the drug is absolutely safe, has a minimum of contraindications and dissolves over time.
Radiesse (Radiesse) is an injectable preparation based on calcium hydroxyapatite, used for facial modeling and wrinkle correction. Calcium hydroxyapatite is similar in composition to bone tissue, it is 100% compatible with the body and dissolves over time.
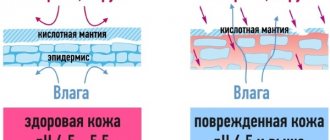
In addition to creating a subcutaneous framework that prevents the skin from “slipping” down, the drugs also trigger the natural process of skin regeneration and stimulate the formation of new collagen. New collagen fibers will make the skin denser and more elastic, providing long-term rejuvenating effects.
[block:webform=client-block-3356]
What is hydroxyapatite?
It has long been believed that hydroxylapatite Ca10(PO4)6(OH)2 is an ideal biocompatible material for restoring damaged bones and teeth. The first documented attempt to use HAp as a bone replacement material dates back to the 1920s. However, the successful use of HAP for these purposes occurred only after 60 years. Hydroxylapatite is perfectly compatible with muscle tissue and skin; Once implanted, it can directly fuse with bone tissue in the body. The high biocompatibility of hydroxyapatite is explained by the crystal chemical similarity of the artificial material to the bone “mineral” of vertebrates.
The name of the mineral comes from the Greek "apatao" - I'm lying, since the beautifully colored natural varieties of apatite were often confused with beryl and tourmaline. Despite the very wide range of colors of natural apatites caused by various impurities, the low hardness (it is the standard value of 5 on the 10-point Mohs scale) does not allow it to be considered as a semi-precious ornamental stone.
It is known that bone mineral contains noticeable amounts (~8% by weight) of carbonate ions; There is also a natural mineral of similar composition - dallit. It is believed that carbonate ions can occupy two different positions in the structure of HAP, replacing hydroxyl and/or phosphate ions to form A- and B-type carbonate hydroxylapatite (CHAP), respectively. Apatite of biological origin belongs to the B-type. The replacement of phosphate ions with carbonate ions leads to a decrease in the crystal size and degree of crystallinity of HAP, and this greatly complicates the study of natural biominerals. An increase in the proportion of carbonate ions in the composition of hydroxyapatite causes natural changes in the equilibrium shape of the crystal. The needle-shaped crystals are “flattened” into plates, which are very similar to the crystallites of apatite existing in the body [5]. Thus, by introducing a small proportion of carbonate ions into the synthesized mineral, it is possible to obtain a material similar to the biogenic one both in chemical composition and geometrically.
An important characteristic of HAP is the stoichiometry of its composition, which is usually expressed by the Ca/P ratio. The variable composition is due to the fact that when synthesizing HAP from solution, it is impossible to protect against H3O+ and HPO42− ions, which can replace Ca2+ and PO43− ions, respectively, in the crystal structure of hydroxylapatite.
Indications for vector lifting
Vector lifting is intended to correct age-related changes between the ages of 30 and 55 years.
The technique allows you to solve problems such as:
- correction of facial contours and elimination of jowls;
- elimination of wrinkles;
- skin tightening by restoring facial proportions;
- harmonization of appearance (correction of the middle third of the face, cheekbones and chin);
- prevention of age-related changes.
Fillers are also suitable for neck correction and hand rejuvenation. To correct more pronounced age-related changes, bioreinforcement can be supplemented with hardware techniques.
Contraindications
- A contraindication is the presence of acute and/or chronic inflammation or infection at the site of the procedure.
- The drug is contraindicated in patients with hypersensitivity to any of the components.
- Contraindicated for patients prone to inflammatory skin reactions or patients with a tendency to hypertrophic scars.
- Do not implant the product into the epidermis or use as a skin substitute. Implanting it into the epidermis or superficial dermis can lead to complications such as fistula formation, infections, extrusions, nodule formation and hardening.
- Not intended for use for the correction of glabellar folds. A higher risk of local necrosis is associated with glabellar infection. Complications associated with other injectable agents indicate that intensive injections into the superficial dermal vessels of the glabellar region may cause retrograde movement into the retinal arteries, which may lead to vascular occlusion.
- Contraindicated in the presence of foreign bodies, for example, liquid silicone or other fractional materials.
- Should not be used in areas with insufficient coverage of healthy, well-vascularized tissue.
- Should not be used in patients with systemic disorders that may result in poor wound healing or tissue damage over the implant.
How is the procedure performed?
If there are no contraindications for injections, the doctor opens the package with filler in front of the patient, treats the skin with an antiseptic and begins administering the drug.
Unpleasant sensations can occur only at the first contact of the skin with the needle, at the depth where the drug is injected - there are no pain receptors. But if the patient wishes, the injection site can be numbed with an anesthetic gel.
The facial bioreinforcement procedure takes 30-60 minutes. After injections, there may be slight swelling and swelling of the tissues, which goes away within 2-3 days.
How is hydroxylapatite used?
There are various methods for the synthesis of hydroxyapatite. They can be roughly divided into high- and low-temperature. High-temperature methods are not of great interest to us, since the materials obtained in this way are practically not bioactive. Low-temperature methods can be divided into two large groups: hydrolysis (including the so-called hydrothermal synthesis methods) and precipitation from solution. The combined method of the so-called sol–gel synthesis is also interesting. In it, the dry residue of the gel undergoes decomposition at a relatively low temperature of 400–700 ° C (compared to high-temperature synthesis). The materials obtained in this way are hard, porous ceramics, whose chemical composition and physical properties resemble bone mineral.
Complex effect
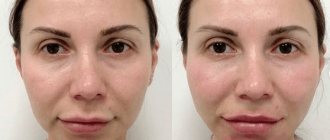
The result of the procedure can be assessed immediately after the procedure; patients note:
- reduction of ptosis;
- increased skin density;
- reduction of jowls;
- significantly less pronounced nasolabial folds;
- clearer oval face.
Long-term results are due to the synthesis of new collagen; its cycle is 21 days, which is why we can talk about the most pronounced result of rejuvenation 3-4 weeks after the procedure.
The effect of bioreinforcement lasts 1-2 years.
PREPARATION FOR PRODUCT OPERATION
Directions for use
The following is required for the subcutaneous injection procedure:
- Radiesse™ Injectable Implant Syringe(s) (sold separately)
- Needle(s) of the required gauge with luer lock connectors (not included in delivery). The required needle size is 25 ga - 27 ga, 1/2 - 11/2 inches. The use of needles smaller than 27 gauge may increase the risk of needle blockage.
1) Prepare the patient for subcutaneous injection using standard techniques. The injection site should be marked with a surgical marker and prepared with an appropriate antiseptic. Local or topical anesthesia at the injection site or sedation should be used at the physician's discretion. After anesthesia, apply ice to reduce swelling/stretching.
2) Before subcutaneous injection, prepare syringes and needles. A new needle should be used for each syringe, or one needle may be attached to each new syringe for the same patient.
Remove the foil packaging from the box. If necessary, the package can be opened and the syringe placed in a sterile field. For sterilization, there is usually a small amount of moisture in the foil bag; this is not a sign of damage.
Advantages of the EMC Clinic
The procedure should be performed exclusively by a dermatocosmetologist. Only a certified specialist knows all the intricacies of the method and is able to correctly determine the depth and location of drug injection.
At the EMC Aesthetic Clinic, specialists masterfully perform the vector skin lifting procedure. We adhere to the principle of naturalness: correction will not distort your facial features, but will only emphasize the beauty of your face and correct age-related changes so that no one will guess about the correction.
This technique combines perfectly with hardware anti-age techniques. During a personal consultation, the doctor will answer all your questions and help you create a comprehensive rejuvenation program.
What problems do drugs based on calcium hydroxyapatite solve?
The drug is recognized as effective for use in the area of nasolabial folds, where the problem of the formation of deep wrinkles is especially acute. Marionette lines that run from the corners of the mouth down to the chin are also treated well with calcium hydroxyapatite. We have already mentioned volumetric modeling; it is also worth noting the effectiveness of the drug for rejuvenating the skin of the hands.
Despite the well-proven effect and the absence of contraindications, the use of drugs with calcium hydroxyapatite is still sometimes fraught with complications. Like all fillers, the drug can lead to infections, swelling and bruising. If the injection depth is incorrectly chosen, the drug can give the effect of a deathly pale face. If the drug is injected into areas not intended for it, for example, into the nasolacrimal groove or lips, lumps, bruises and irregularities may form that will take a very long time to resolve. Injection too close to the surface may result in the appearance of white streaks that will remain until the drug dissolves on its own.
Thus, in the hands of an illiterate specialist, the drug can lead to disastrous consequences that are difficult to get rid of. And, on the contrary, when used by an experienced certified physician, calcium hydroxyapatite will help to successfully restore lost volumes and prolong youth.
FAQ
Is vector lifting painful?
For those patients who are not seeing a cosmetologist for the first time and know first-hand what biorevitalization and contour plastic surgery are, vector lifting will seem a much more comfortable procedure. Minor pain is present only when the skin is pierced. Direct reinforcement is a completely painless technique, since there are no pain receptors at this depth. During the procedure, no more than ten tiny punctures are performed, optionally using an anesthetic cream, which we use to treat the areas where the drug is injected. The gel is injected vectorially with a special needle or cannula, which is why the procedure is called vector lifting. Most often, bioreinforcement is performed with Restylane Vital and Radiesse.
What is the difference between vector lifting and contour plastic surgery?
The main goal of vector lifting is to return the tissue to its original place by creating a frame and remodel the clear oval of the face. Contour plastic surgery is aimed at replenishing lost volume in the area of the cheekbones, cheeks, and temples.
How does the body react to calcium phosphate ceramics?
Bioactivity is a complex characteristic of materials compatible with the body, which takes into account, in addition to the impact on the biological processes of cell growth and differentiation, also:
- the rate of dissolution of the material in environments created by certain groups of cells (bioresorbability);
- the rate of deposition of material from the interstitial fluid onto the surface of the material.
Among the requirements that apply to bioactive materials used in medical practice to restore the integrity of bone tissue, the first place is occupied by a relatively high dissolution rate (of the order of tens of microns per year) - the so-called bioresorbability. The surface plays an active role in the biochemical reactions occurring at the bone/implant interface with the participation of cells specific to the process of osteosynthesis. When talking about the rate of resorption of material located in the interstitial fluid, it is customary to compare new materials with those already used in medicine - ceramics based on hydroxyapatite or β-tricalcium phosphate. Coarse-crystalline ceramics based on HAp are resorbed slowly, so inclusions of artificial material can be detected in the bone even after many years. Ceramics produced using β-Ca3(PO4)2 dissolve so quickly that growing bone does not have time to fill the resulting cavities. The rate of dissolution of the material depends on many factors: surface area, structure, composition, defectiveness of the material. These characteristics determine the body's response to a foreign implant. Bioactive materials are characterized by rapid fusion with bone tissue through the formation of an intermediate layer of HAP, which is formed in two possible ways:
- Dissolution of calcium phosphate - precipitation of hydroxyapatite.
- Precipitation of HAP from a supersaturated solution in tissue fluid.
An important procedure for assessing bioactivity involves in vivo testing. It is expensive and time-consuming, and also carries risks. However, active development of methods is underway that makes it possible, already at an early stage of preclinical testing, to rank materials according to the degree of bioactivity in the course of relatively simple in vitro experiments simulating processes in the human body - the dissolution of the material and the deposition of HAP on the surface of the material from solutions similar to body fluids.
The study of the bioactivity of materials is carried out using a solution that imitates the ionic composition of human interstitial fluid. Compact samples of the test material are placed in the solution for several days at 37 °C. The process of deposition of carbonate hydroxylapatite from a model solution onto the surface of the material is controlled by X-ray phase analysis, IR spectroscopy and scanning electron microscopy.
There is a need to regulate the bioresorbability of artificial materials, depending on their purpose. This possibility exists due to the different properties of materials with different compositions. To make a sample more resorbable, it is necessary to increase the proportion of carbonate and silicate ions in the crystal lattice of the material.
Figure 3. Openwork layer of partially resorbed ceramic. Scanning electron microscope image. Shown here is a fragment of material subjected to dissolution in a model solution in vitro. On the right you can see what the material was like before resorption began.
Silicon-containing material exhibits the best bioactivity in such studies. Silanol (—SiOH) groups are formed on its surface, actively participating in the mineralization of the outer layer of the implant. Such a material intensively exchanges ions with the solution: silanol groups firmly bind calcium ions, promoting the formation of a layer of amorphous calcium phosphate on the surface, the separation and crystallization of which leads to the formation of an openwork layer consisting of HAP particles ~10 nm in size (Fig. 3). Differences in the thickness of such a layer can serve as a measure of the bioactivity of the material: the thicker it is, the easier it will be for the bone to integrate this material into its structure.
Another of the most important properties of modern implantation materials is osteoinductivity—the ability to support the vital activity of osteoblasts and stimulate ectopic (outside the bone) de novo bone tissue formation. This is the most important property for artificial implants. The fact is that to initiate bone formation around the implant, a microenvironment with particles of living bone is necessary. The newly formed bone gradually fuses with the surrounding implanted particles, “jumping” from one to another.
It is believed that the most active from the point of view of osteosynthesis is the amorphous modification of hydroxyapatite. However, sufficiently crystalline HAP with crystallite sizes approaching those of crystals in bone tissue (20–40 nm3) can show results an order of magnitude higher than the amorphous cements currently used [6].
Bioinert materials do not affect the osteosynthesis process in any way. On the surface of implants made from them, fibrous tissue is formed, which prevents the formation of a connection between the implant and the bone. There is a significant likelihood of rejection of such materials by the body, often accompanied by inflammatory processes. However, it is not yet possible to completely abandon these materials, since they are cheap and easy to process. The main problems that are solved when designing implants from bioinert materials are bringing the elastic characteristics of the implant closer to the characteristics of bone, as well as reducing the rate of corrosion processes.
Unlike bioinert synthetic materials based on polymers and metals, ceramics based on calcium phosphates are biocompatible and bioactive, and therefore are the most promising material for bone implants. Its main disadvantage is fragility. So far, the best solution is to use composites made of metals or polymers coated with calcium phosphate ceramics (Fig. 4). They provide good integration of the material into bone tissue, preventing the formation of fibrous tissue around the bioinert metal. Over time, the prosthesis will fuse very firmly with the surrounding bone, which will replace the layer of HAP. The failure rate of such prostheses is significantly lower than that of metal and plastic analogues.
Figure 4. Bioactive ceramic coating on a hip joint prosthesis. a — Porous structure of the ceramic coating. b — X-ray image of the prosthesis implanted in place of the hip joint. The prosthesis itself is made of titanium and polymers.
[9] and Insurers pay twice as much as hospitals for hip and knee implants