pharmachologic effect
Xeomin acts selectively on peripheral cholinergic nerve endings, inhibiting the release of acetylcholine. Entry into cholinergic nerve endings occurs in three stages: binding of the molecule to external membrane components, internalization of the toxin by endocytosis, and translocation of the endopeptidase domain of the toxin from the endosome to the cytosol. In the cytosol, the endopeptidase domain of the toxin molecule selectively cleaves SNAP-25, an important protein component of the mechanism that controls the membrane movement of exo-vesicles, thus stopping the release of acetylcholine. The end effect is relaxation of the injected muscle.
The effect of the drug begins within four to seven days after the injection. The effect of each procedure usually lasts three to four months, although it can last significantly longer or shorter.
Botulinum toxin Xeomin (Xeomin)
Botulinum toxin Xeomin is a highly purified botulinum toxin type A. A drug with increased safety is used to correct facial wrinkles on the forehead, between the eyebrows, around the eyes and mouth. Xeomin effectively smooths out wrinkles, while preserving natural facial expressions. The
manufacturer of the drug Xeomin is the company Merz, a successful German pharmaceutical company. The drug is safe and easy to use; it can be stored at room temperature for three years.
Botulinum toxin Xeomin is a second generation botulinum toxin type A drug, the great advantage of which is that it is a pure neurotoxin, free of complex proteins. Since 2001, the drug has been produced by the pharmaceutical company Merz. Today the drug is registered and used in many European countries.
Contraindications
The drug is contraindicated in people with allergies to the components of the drug, with disorders of neuromuscular transmission (masthenia gravis, Lambert-Eaton syndrome, prescribed with caution for amyotrophic lateral sclerosis, for neurological diseases resulting from degeneration of motor neurons and other diseases with disorders of neuromuscular transmission) . The drug is not administered at elevated temperatures and acute infectious or non-infectious diseases.
The drug is contraindicated during pregnancy and lactation.
The drug is not administered to children and adolescents under 18 years of age.
Botulinum therapy is a unique technique for restoring youth
Can! Botulinum therapy helps get rid of unwanted signs of aging on the face and décolleté. For many years, only one drug was used for this procedure - the well-known Botox. How does it work?
Botulinum toxin (a specific poison) is injected under the skin, which paralyzes the muscles, they do not contract, wrinkles are smoothed out, and the face looks more youthful. However, medicine does not stand still, and doctors decided to develop a new pharmaceutical product.
Directions for use and doses
V/m. The drug should only be administered by doctors who have special training and experience in handling botulinum toxin and electromyography equipment. The doctor sets the dosage and number of injection sites into the muscle individually for each patient.
Blepharospasm: after dissolution, Xeomin is administered with a sterile 27–30 G needle.
The recommended initial dose is 1.25–2.5 units (0.05–0.1 ml) at each injection site; the drug is injected into the medial and lateral parts of the circular muscle of the eye (m. orbicularis oculi) of the upper eyelid and into the lateral part of the circular muscle of the eye of the lower eyelid.
If vision is impaired due to spasms in the forehead, lateral orbicularis oculi muscle, and upper face, additional injections may be given to these areas. The effect of the drug begins, on average, within 4 days after injection. The effect of each procedure usually lasts 3-4 months, although it can last significantly longer or shorter.
If the effect of the initial dose was insufficient (duration
The dosages recommended for Xeomin cannot be used for conversion when using other drugs containing botulinum toxin.
Since the drug does not contain antimicrobial agents, it is recommended to use it immediately after dissolution. If necessary, the dissolved drug can be stored in the original vial for up to 24 hours in the refrigerator at a temperature of 2 to 8 ° C, provided that the dissolution was carried out under aseptic conditions.
Blepharospasm:
After dissolution, Xeomin is administered with a sterile 27-30 G needle. The recommended initial dose is 1.25-2.5 units (0.05-0.1 ml) at each injection site; the drug is injected into the medial and lateral parts of the circular muscle of the eye (m. orbicularisoculi) of the upper eyelid, and into the lateral part of the circular muscle of the eye of the lower eyelid.
If vision is impaired due to spasms in the forehead, lateral orbicularis oculi muscle, and upper face, additional injections may be given to these areas. The effect of the drug begins, on average, within four days after the injection. The effect of each procedure usually lasts three to four months, although it can last significantly longer or shorter.
If the effect of the initial dose was insufficient (duration less than two months), with repeated procedures the dose of the drug can be doubled. The initial dose should not exceed 25 units per eye. No dose greater than 5.0 units should be injected into each site. When treating blepharospasm, the total dosage over twelve weeks of treatment should not exceed 100 units.
Spastic torticollis: When treating spastic torticollis, the dosage should be selected for each patient individually, depending on the position of the neck and head, the location of pain, muscle volume (hypertrophy, atrophy), and the patient’s body weight.
In treatment practice, the maximum dose of the drug during one procedure should usually not exceed 200 units, but dosages up to 300 units are possible. A dose of the drug exceeding 50 units should not be administered to the same place.
Treatment for spasmodic torticollis includes injections into the sternocleidomastoid muscle, levator scapulae muscle, scalene muscle, splenius muscle, and/or trapezius muscle(s).
Injections into both sternocleidomastoid muscles should not be given as this increases the risk of adverse drug effects (particularly dysphagia) that occur with bilateral drug injections into this muscle or at doses greater than 100 units.
For injections into superficial muscles, 25, 27 and 30 G needles are used, and for deep muscles, a 22 G needle is used.
In spastic torticollis, electromyography may be necessary to determine the muscles involved. Injecting into several places allows the drug to evenly cover muscle areas affected by dystonia (especially when injecting into large muscles). The optimal number of injection sites depends on the size of the muscle. The effect of the drug begins, on average, within seven days after the injection. The effects of each treatment last approximately three to four months, but may last significantly longer or shorter. The interval between procedures should be at least ten weeks.
Arm spasticity after stroke:
The drug is administered using suitable sterile needles (for example, for superficial muscles use 26 G needles with a diameter of 0.45 mm and a length of 37 mm, for deep muscles use 22 G needles with a diameter of 0.7 mm and a length of 75 mm).
Electromyography may be necessary to determine the muscles involved. Injecting into several places allows the drug to evenly cover muscle areas affected by dystonia (especially when injecting into large muscles).
When treating spasticity, the dosage should be selected individually for each patient, depending on the size, number and location of the muscles involved, the severity of static, and the presence of local muscle weakness.
Suggested dosages are presented in the table:
| Solvent volume, ml | U/0.1 ml |
| 0,5 | 20 |
| 1 | 10 |
| 2 | 5 |
| 4 | 2,5 |
| 8 | 1,25 |
| Clinical symptom Muscle | Units |
| Hand bent at the wrist | |
| Flexor carpi radialis | 50 |
| Flexor carpi ulnaris | 40 |
| clenched fist | |
| Flexor digitorum superficialis | 40 |
| Flexor digitorum profundus | 40 |
| Arm bent at the elbow | |
| Brachioradial | 60 |
| Biceps | 80 |
| Shoulder | 50 |
| Pronated forearm | |
| Pronator quadratus | 25 |
| Pronator teres | 40 |
| Thumb adducted to palm | |
| Flexor pollicis longus | 20 |
| Adductor pollicis muscle | 10 |
| Flexor pollicis brevis/oppons pollicis muscle | 10 |
When treating spasticity, the recommended dosage per course of treatment is 170 - 400 IU, depending on the location of the spasmed muscles.
Improvement occurs within the first 2 weeks, reaching a maximum by the 4th week. The effect usually lasts 12 weeks.
Hyperkinetic folds (expression wrinkles) of the face:
The use of the drug for the treatment of facial wrinkles in persons under 18 years of age and over 65 years of age is not recommended due to the lack of clinical experience.
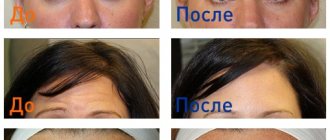
Vertical wrinkles between the eyebrows (glabellar wrinkles): the recommended injection volume is 0.1 ml (4 units) - injected into each of 5 areas: 2 injections per m. corrugator on both sides and 1 injection into the m.procerus, which corresponds to a total dose of 20 units. In some cases, it is possible to increase the total dose to 30 units.
Before and during injection, apply pressure with the thumb and forefinger below the upper edge of the orbit to prevent diffusion of the solution into this area. During injection, the needle should be directed upward and medially. To reduce the risk of eyelid ptosis, injections should be avoided near the levator palpebrae superioris muscle and the insertion of the orbicularis oculi muscle. Injections into m. The corrugator should be carried out into the medial part of the muscle and into the central part of the muscle belly, at least 1 cm above the upper edge of the orbit.
Reduction in the severity of vertical wrinkles between the eyebrows (glabellar wrinkles) usually develops within 2-3 days, the maximum effect is observed by the 30th day, and lasts up to 4 months after the injection. The intervals between injections should not be less than 3 months. If the treatment result is unsatisfactory, alternative treatment methods are used.
How does it work?
Let's consider a typical situation. An N-age woman decided to “deceive nature and those around her.” Upon arrival at a specialized institution, she was offered several injections for wrinkles. Naturally, the client asked: how does this drug act on the body and what will the results be?
How should a specialist explain this in simple, non-scientific language? Xeomin is injected into the muscle area. The molecules penetrate the nerve fibers and block the contraction of muscle fibers. Temporary paralysis of the nerve endings occurs, wrinkles are smoothed out.
After 3-4 months, the innervation is restored and the drug ceases its effect.
Side effects
Adverse reactions are classified by frequency of occurrence: very often (≥ 1/10), often (≥1/100 to Blepharospasm:
Common: ptosis (6.1%), dry eyes (2.0%).
Uncommon: paresthesia, conjunctivitis, dry mouth, skin rash, headache, muscle weakness.
In addition, when using a similar drug containing botulinum toxin type A, and used in clinical trials along with Xeomin, the following side effects were observed. They are also possible when using Xeomin.
Common: superficial keratitis, lagophthalmos, skin irritation, photophobia, lacrimation.
Uncommon: keratitis, ectropia, diplopia, dizziness, diffuse skin rash/dermatitis, entropion, focal facial paralysis, facial weakness, fatigue, visual disturbances, blurred vision.
Rare: local swelling of the skin of the eyelid.
Very rare: acute angle-closure glaucoma, corneal ulceration.
Spasmodic torticollis:
Common: dysphagia (10%), muscle weakness (1.7%), back pain (1.3%). Uncommon: inflammation or pressure at the injection site, headache, asthenia, generalized increased sweating, tremor, hoarseness, colitis, vomiting, diarrhea, dry mouth, bone pain, myalgia, skin rashes, itching, peeling of the skin, pain in eyes.
In addition, when using a similar drug containing botulinum toxin type A, and used in clinical trials along with Xeomin, the following side effects were observed. They are also possible when using Xeomin.
Very common: pain at the injection site.
Common: dizziness, high blood pressure, numbness at the injection site, general weakness, flu-like symptoms, general malaise, dry mouth, nausea, headache, muscle stiffness at the injection site, irritation at the injection site, rhinitis, upper respiratory tract infections.
Uncommon: shortness of breath, diplopia, fever, ptosis, speech disorders.
The severity of dysphagia varies from mild to severe, with the possibility of aspiration; In rare cases, this requires medical attention. Dysphagia may persist for two to three weeks after injection, but a case lasting 3 months has been reported. Dysphagia develops in a dose-dependent manner; According to clinical studies, dysphagia is rare if the total dose of the drug does not exceed 200 units per procedure.
Arm spasticity after stroke:
Common: headache, sensory disturbance, feeling of heat.
In addition, when using a similar drug containing botulinum toxin type A, and used in clinical trials along with Xeomin, the following side effects were observed. They are also possible when using Xeomin.
Common: hypertonicity, ecchymosis, local pain, muscle weakness, irritation or hemorrhage at the injection site.
Uncommon: depression, sleep disturbances, paresthesia, incoordination, amnesia, vertigo, orthostatic hypotension, nausea, dermatitis, itching, rash, arthralgia, bursitis, asthenia, pain, hypersensitivity at the injection site, anxiety, peripheral edema.
Some side effects may be related to the underlying disease.
Hyperkinetic folds (expression wrinkles) of the face:
Usually, undesirable effects are observed in the first week after using the drug and are temporary. Undesirable effects may be related to the active substance and/or the administration procedure.
The expected pharmacological effect of botulinum toxin is localized muscle weakness. Ptosis of the eyelids can be caused by the injection technique and is associated with the pharmacological action of the drug.
As with any injection, local pain, tenderness, itching, swelling and/or hematoma may occur; It is also possible to develop transient vasovagal reactions, such as fainting, circulatory disorders, nausea or tinnitus associated with anxiety before the injection.
The following adverse reactions have been reported with the use of the drug:
Common: skin itching, headache, muscle dysfunction at the injection site, feeling of heaviness.
Uncommon: flu-like symptoms, feeling of tension at the injection site, bronchitis, nasopharyngitis, feeling of "nodules in the skin", swelling of the eyelids, ptosis, blurred vision, raised eyebrows, nausea, muscle twitching and spasms, local weakness of the facial muscles.
In addition, the comparator drug, which contains a conventional botulinum toxin type A complex and was used in several clinical studies (data collected to date suggests equal activity for both drugs), is known to develop the following adverse effects, which are likely to occur with Xeomina.
Uncommon: infection, paresthesia, dizziness, blepharitis, eye pain, dry mouth, photosensitivity, dry skin.
Common side effects:
The information presented below is based on data on the effects of other products containing botulinum toxin type A.
Information about severe negative effects that may be associated with damage to the cardiovascular system - such as arrhythmia and myocardial infarction, including death - is extremely rare. Whether these deaths were caused by botulinum toxin type A injections or concomitant cardiovascular diseases is not certain. One case of anaphylactic shock has been reported after administration of a drug containing botulinum toxin type A.
Side effects such as exudative erythema multiforme, urticaria, psoriasis-like rashes, itching and allergic reactions are noted, but their dependence on the action of a complex drug containing botulinum toxin type A has not been confirmed.
Sometimes after injection of botulinum toxin type A, changes in the electrophysiological background were noted in some distant muscles; this side effect is not associated with muscle weakness or other electrophysiological abnormalities.
What's in reality?
Having studied the opinions of women who used this drug, it is impossible to draw an unambiguous conclusion: some really liked Xeomin, others did not at all.
Several conclusions can be absolutely certain
- not everyone is satisfied with the slow action of the drug;
- women do not see ANY results after 10 days from injections (information from forums on this topic);
- wrinkles disappear within 3–4 months.
For comparison, a drug of the same series, Dysport, is valid for 12 months; as a result, the price of Xeomin will be more expensive. Either inject Dysport once a year, or Xeomin 3-4 times.
However, there is also “the other side of the coin:
Xeomin, according to reviews from cosmetologists, is a more gentle drug - its formula is softer, there is a lower risk of allergic reactions, and therefore the result is not so durable.
Here every lady chooses her own. If you want to remove wrinkles for a long time and are not afraid of possible reactions on the body (don’t forget about the “stony face” in the end), Xeomin will not suit you - get Botox injections.
If you are not bothered by the gradual smoothing out of wrinkles, and you value safety, then the new German drug is ideal for you.
After Botox injections, Xeomin will not give any results (again, according to reviews).
Overdose
High doses of Xeomin can lead to the development of severe muscle paralysis in areas remote from the injection sites (in particular: general weakness, ptosis, diplopia, difficulty speaking and swallowing, as well as paralysis of the respiratory muscles, leading to the development of aspiration pneumonia).
In case of overdose, hospitalization with general supportive measures is necessary. In case of paralysis of the respiratory muscles, intubation and artificial ventilation of the lungs are necessary until the condition normalizes.
Features of the drug
Botulinum therapy is a recognized remedy for facial wrinkles. The safety of botulinum therapy has been fully proven, and its effect is achieved almost instantly and lasts for several months.
Xeomin has serious advantages compared to other botulinum toxin A preparations:
- The highest degree of botulinum toxin purification eliminates the presence of protein compounds. Other drugs contain some protein. This is what explains the reaction from the immune system in the form of redness and swelling at the injection sites. These phenomena occur relatively rarely, and the use of Xeomin eliminates them completely.
- The high concentration of botuloxin in Xeomin allows the use of lower dosages.
- Xeomin makes it possible to act selectively on muscles. Thanks to this, only those facial muscles that create wrinkles are blocked. Those muscles that determine the individual features of your face, its expressiveness and liveliness will not change at all.
- The high purity of Xeomin guarantees its exceptional hypoallergenicity.